Lambert–Eaton myasthenic syndrome
From Wikipedia, the free encyclopedia
Jump to: navigation,
search
|
Lambert–Eaton myasthenic syndrome |
|
|
Classification and external resources |
|
|
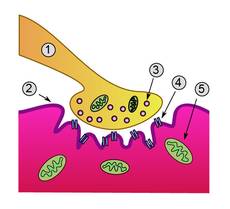
Neuromuscular junction. Lambert-Eaton myasthenic
syndrome is caused by autoantibodies to the presynaptic membrane. Myasthenia
gravis is caused by autoantibodies to the postsynaptic acetylcholine
receptors. |
|
Lambert–Eaton
myasthenic syndrome (LEMS
Lambert-Eaton syndrome or Eaton–Lambert syndrome) is a rare autoimmune
disorder that is characterised by muscle weakness
of the limbs. It is the result of an autoimmune reaction in which antibodies are
formed against presynaptic voltage-gated calcium channels,
and likely other nerve terminal proteins, in the neuromuscular junction (the connection
between nerves and
the muscle that
they supply).[1]
The prevalence is 3.4 cases per million.[2]
Around 60% of those with LEMS have an underlying malignancy, most
commonly small cell lung cancer; it is therefore
regarded as a paraneoplastic syndrome (a condition that
arises as a result of cancer elsewhere in the body).[3]
People who
develop LEMS are usually over 40, although it may occur at any age. The
diagnosis is usually confirmed with electromyography
and blood
tests; these also distinguish it from myasthenia
gravis (MG), a related autoimmune neuromuscular disease.[1]
If the
disease is associated with cancer, direct treatment of the cancer often
relieves the symptoms of LEMS. Other treatments often used are steroids,
azathioprine
and intravenous immunoglobulin, which
suppress the immune system, and pyridostigmine
and 3,4-diaminopyridine, which enhance the
neuromuscular transmission. Occasionally, plasma
exchange is required to remove the antibodies.[1]
Contents
Signs and symptoms
The weakness
of LEMS typically involves the legs and arms. Leg involvement is more striking
than in myasthenia gravis. The proximal muscles (those
closest to the trunk) are predominantly affected; this leads to difficulties
climbing stairs and rising from a sitting position. Physical
exercise and high temperatures can worsen the symptoms. Weakness of the bulbar muscles (supplying the mouth and throat)
is occasionally encountered.[1]
Weakness of the eye muscles is uncommon. Some may have double vision, drooping
of the eyelids and difficulty swallowing,[1]
but generally only together with leg weakness; this distinguishes LEMS from
myasthenia gravis, in which eye signs are much more prominent.[3]
In the advanced stages of the disease, weakness of the respiratory muscles may occur.[1]
Some may also experience problems with coordination (ataxia).[4]
Three
quarters of people with LEMS also have disruption of the autonomic nervous system. This may be
experienced as a dry mouth, constipation,
blurred
vision, impaired sweating, and orthostatic hypotension (falls in blood pressure
on standing, potentially leading to blackouts). Some report a metallic taste in the
mouth.[1]
On neurological examination, the weakness
demonstrated with normal testing of power is often less severe than would be
expected on the basis of the symptoms. Strength improves further with repeated
testing, e.g. improvement of power on repeated hand grip (a phenomenon known as
"Lambert's sign"). At rest, reflexes are
typically reduced; with muscle use, reflex strength increases. This is a
characteristic feature of LEMS. The pupillary light reflex may be sluggish.[1]
In LEMS
associated with lung cancer, most have no suggestive symptoms of cancer at the
time, such as cough,
coughing
blood and unintentional weight loss.[3]
It has been suggested that LEMS associated with lung cancer is more severe.[4]
Causes
LEMS is
often associated with lung cancer (50–70%),[1]
making LEMS a paraneoplastic syndrome.[4]
Of the people with small cell lung cancer, 1–3% have LEMS.[3]
In most of these cases, LEMS is the first symptom of the lung cancer, and it is
otherwise asymptomatic.[3]
LEMS may
also be associated with autoimmune diseases, such as hypothyroidism
(an underactive thyroid gland) or diabetes mellitus type 1.[1][5]
Myasthenia gravis, too, may happen in the presence of tumors (thymoma, a tumor
of the thymus in
the chest); people with MG without a tumor and people with LEMS without a tumor
have similar genetic variations that seem to predispose them to these diseases.[3]
HLA-DR3-B8 (an HLA subtype), in particular, seems to
predispose to LEMS.[5]
Mechanism
In normal
neuromuscular function, a nerve
impulse is carried down the axon (the long projection of a nerve cell) from
the spinal
cord. At the nerve ending in the neuromuscular junction, where the impulse is
transferred to the muscle cell, the nerve impulse leads to the opening of voltage-gated calcium channels
(VGCC), the influx of calcium ions into the nerve terminal, and the
calcium-dependent triggering of synaptic vesicle fusion with plasma membrane.
These synaptic vesicles contain acetylcholine,
which is released into the synaptic cleft and stimulates the acetylcholine receptors on the muscle. The
muscle then contracts. [1]
In LEMS,
antibodies against VGCC, particularly the P/Q-type VGCC, decrease the amount of calcium
that can enter the nerve ending, hence less acetylcholine can be released from
the neuromuscular junction. Apart from skeletal
muscle, the autonomic nervous system also requires acetylcholine
neurotransmission; this explains the occurrence of autonomic symptoms in LEMS.[1][3]
P/Q voltage-gated calcium channels are also found in the cerebellum,
explaining why some experience problems with coordination.[4][5]
The antibodies bind particularly to the part of the receptor known as the
"domain III S5–S6 linker peptide".[5]
Antibodies may also bind other VGCCs.[5]
Some have patients have antibodies that bind synaptotagmin,
the protein sensor for calcium-regulated vesicle fusion.[5]
Many people with LEMS, both with and without VGCC antibodies, have detectable
antibodies against the M1 subtype of the acetylcholine
receptor; it is thought that their presence participates in a lack of
compensation for the weak calcium influx.[5]
Apart from
the decreased calcium influx, there is also a disruption of active zone vesicle
release sites, which is also thought to be antibody-dependent since LEMS
patients have antibodies to components of these active zones (including
voltage-dependent calcium channels). Together, these abnormalities lead to the
decrease in muscle contractility. Repeated stimuli over a period of about 10
seconds eventually lead to sufficient delivery of calcium, and an increase in
muscle contraction to normal levels, which can be demonstrated on
electromyography by increasing amplitude of repeated compound muscle action potentials.[1]
It has been
shown that the antibodies found in LEMS associated with lung cancer also bind
to calcium channels in the cancer cells, and it is presumed that the antibodies
originally develop as a reaction to these cells.[1]
It has been suggested that the immune reaction to the cancer cells suppresses
their growth and improves the prognosis from the cancer.[3][5]
Diagnosis
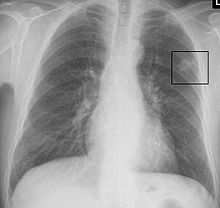
Chest X-ray showing a tumor in the
left lung (right side of the image)
The
diagnosis is usually made on electromyography
(EMG), which is one of the standard tests in the investigation of otherwise
unexplained muscle weakness. This involves the insertion of small needles into
the nerves supplying several muscles, administering small electrical impulses
through these needles, and measuring the electrical response of the muscle in question.
Two EMG investigations can be characteristic in LEMS: compound motor action
potentials (CMAP) and single-fiber examination.[1]
CMAP shows
small amplitudes but normal latency and conduction velocities. If repeated
impulses are administered (two per second or 2 Hz), it is normal for CMAP
amplitudes to become smaller as the acetylcholine in the motor end plate is
depleted. In LEMS, this decrease is larger than observed normally. Eventually,
stored acetylcholine is made available, and the amplitudes increase again. In
LEMS this remains insufficient to reach a level sufficient for transmission of
an impulse from nerve to muscle; all can be attributed to insufficient calcium
in the nerve terminal. A similar pattern is witnessed in myasthenia gravis. In
LEMS, in response to exercising the muscle, the CMAP amplitude increases
greatly (over 200%, often much more). This also occurs on the administration of
a rapid burst of electrical stimuli (20 impulses per second for ten seconds).
This is attributed to the influx of calcium in response to these stimuli.[1][3]
On single-fiber examination, features may include increased jitter (seen in
other diseases of neuromuscular transmission) and blocking.[1]
Blood tests
may be performed to exclude other causes of muscle disease (elevated creatine
kinase may indicate a myositis, and abnormal thyroid function tests may indicate thyrotoxic myopathy). Antibodies against
voltage-gated calcium channels can be identified in 85% of people with EMG
confirmed LEMS.[1]
Once LEMS is diagnosed, investigations such as a CT scan of the chest are usually
performed to identify any possible underlying lung tumors. 50–60% of these are
discovered immediately after the diagnosis of LEMS. The remainder is diagnosed
later, but usually within two years and typically within four years.[3]
As a result, scans are typically repeated every six months for the first two
years after diagnosis.[1]
While CT of the lungs is usually adequate, a positron emission tomography (PET)
scan of the body may also be performed to search for an occult tumour,
particularly of the lung.[6]
Treatment
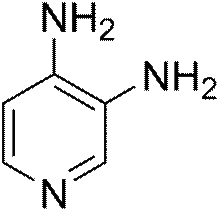
Molecular structure of
3,4-diaminopyridine, a commonly used drug treatment for LEMS.
If LEMS is
caused by an underlying cancer, treatment of the malignancy usually leads to
resolution of the symptoms.[1]
Treatment usually consists of chemotherapy,
with radiation therapy in those with limited disease.[3]
The three
treatment modalities aimed at improving LEMS symptoms directly are improving
the neuromuscular conduction and suppression of the immune system. Two classes
of medication are used to improve neuromuscular transmission. Pyridostigmine
decreases the degradation of acetylcholine after release into the synaptic cleft,
and thereby improves muscle contraction. In LEMS, the potassium
channel blocker 3,4-diaminopyridine (amifampridine) is also used. It delays
the repolarization of nerve terminals after a discharge, thereby allowing more
calcium to accumulate in the nerve terminal.[1][3]
An older agent, guanidine, causes many side-effects and is not recommended.
4-Aminopyridine
(dalfampridine), an agent related to 3,4-aminopyridine, causes more
side-effects than 3,4-DAP and is also not recommended.[3]
Immune
suppression tends to be less effective than in other autoimmune diseases.
Prednisolone (a glucocorticoid or steroid) suppresses the immune response, and
the steroid-sparing agent azathioprine may replace it once therapeutic effect
has been achieved. Intravenous immunoglobulin (IVIG) may be
used with a degree of effectiveness. Plasma exchange (or plasmapheresis), the
removal of plasma proteins such as antibodies and replacement with normal
plasma, may provide improvement in acute severe weakness. Again, plasma
exchange is less effective than in other related conditions such as myasthenia
gravis, and additional immunosuppressive medication is often needed.[1]
According to a systematic review by the Cochrane Collaboration, the best evidence in
the treatment of LEMS exists for 3,4-aminopyridine and IVIG.[7]
History
Anderson and
colleagues from St Thomas' Hospital, London, were the first to
mention a case with possible clinical findings of LEMS in 1953,[8]
but Lambert, Eaton and Rooke at the Mayo Clinic
were the first physicians to substantially describe the clinical and
electrophysiological findings of the disease in 1956.[9][10]
In 1972, the clustering of LEMS with other autoimmune diseases led to the
hypothesis that it was caused by autoimmunity.[11]
Studies in the 1980s confirmed the autoimmune nature,[5]
and research in the 1990s demonstrated the link with antibodies against
P/Q-type voltage-gated calcium channels.[1][12]